Semaglutide CAS 910463-68-2 Purity ≥99.0% (HPLC) Type 2 Diabetes Treatment
Shanghai Ruifu Chemical Co., Ltd. is the leading supplier of Semaglutide (CAS: 910463-68-2) with high quality, can provide worldwide delivery, competitive price, excellent service.
Purchase Semaglutide or other peptides, please contact us by e-mail: alvin@ruifuchem.com
| Peptide Name | Semaglutide |
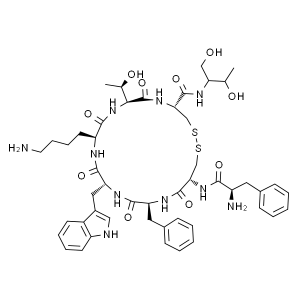
| Peptide Sequence | H-His-Aib-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Gln-Ala-Ala-Lys(C18diacid-γ-Glu-OEG-OEG)]-Glu-Phe-Ile-Ala-Trp-Leu-Val-Arg-Gly-Arg-Gly-OH; H-Aib-EGTFTSDVSSYLEGQAAK(AEEA-AEEA-γ-glu-18Otc)EFIAWLVRGRG |
| Synonyms | Ozempic® (Injection) and Rybelsus® (Oral); NN9535, OG217SC, NNC 0113-0217 |
| Stock Status | In Stock, Commercial Production 15kg/Month, 1kg in Stock |
| CAS Number | 910463-68-2 |
| Molecular Formula | C187H291N45O59 |
| Molecular Weight | 4113.64 g/mol |
| Purity | ≥99.0% (HPLC) (or Refer to the Certificate of Analysis) |
| Formulation | Lyophilized Powder For Reconstitution |
| Peptide Reconstitution | 1mg Peptide is Soluble in 0.6ml H2O+0.3ml ACN+0.1ml NHH2O |
| Storage | After Reconstitution store at 2℃ - 8℃ |
| Packaging Container | Glass Freeze-Dried Bottle |
| Shelf Life | >2 Years if Stored Properly |
| Store Under Inert Gas | Store Under Inert Gas |
| Grade | Pharmaceutical Grade, Produced in GMP Condition Workshop |
| Originator / First Marketed by | Novo Nordisk |
| Original FDF | RTU Solution for Subcutaneous Injection |
| Other FDF | Oral Tablets |
| Mode of Action | Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists |
| Indications | Obesity, Type 2 Diabetes Mellitus |
| Caution | Not For Human Use. For Research Use Only. |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White Lyophilized Powder | White Lyophilized Powder |
| Purity / Analysis Method | ≥99.0% (HPLC) | 99.12% |
| MS Spectrum | Consistent With Structure | Consistent |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: 10mg/vial, 50mg/vial, 100mg/vial, 1g/vial, or according to customer's requirement.
Storage Condition: Keep the vial tightly closed. Store in a cool, dry and at 0~4℃ for short term (days to weeks) or -20℃ for long term (months to years).
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Semaglutide (CAS: 910463-68-2) (sold under brand names Ozempic® (Injection) and Rybelsus® (Oral) is a recombinant DNA produced polypeptide analogue of human glucagon-like peptide-1 (GLP-1) which is used in the therapy of type 2 diabetes.
Semaglutide is a long-acting GLP-1 analogue, is a glucagon-like peptide-1 (GLP-1) receptor agonist. Semaglutide has the potential for type 2 diabetes treatment. It reduces blood sugar via increasing the production of insulin. It enhances insulin secretion and reduces appetite, leading to improved glycemic control and weight loss.
On Oct 18, 2017, Novo Nordisk received positive 16-0 vote from FDA Advisory Committee in favor of approval for Semaglutide.
Subcutaneous formulation is still patented until 2027-12-05. Oral formulation is patented until 2029-09-20.
-

Semaglutide CAS 910463-68-2 Purity ≥99.0% (HPLC...
-

Tirzepatide CAS 2023788-19-2 Purity (HPLC) ≥99....
-
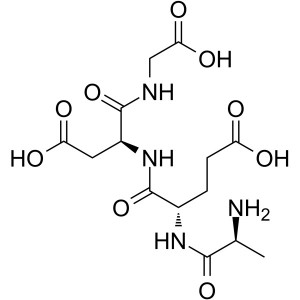
Epitalon CAS 307297-39-8 Purity ≥98.0% (HPLC) H...
-
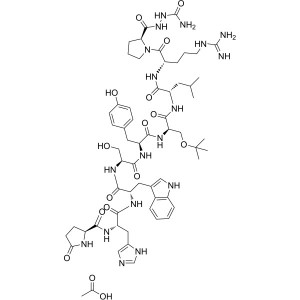
Goserelin Acetate CAS 145781-92-6 Purity >99.0%...
-
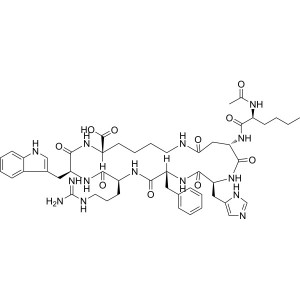
Bremelanotide (PT-141) CAS 189691-06-3 Purity ≥...
-
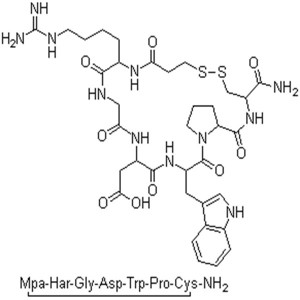
Eptifibatide Acetate CAS 148031-34-9 (Free Base...
-
Histrelin Acetate CAS 76712-82-8 Peptide Purity...
-
Desmopressin Acetate CAS 16789-98-3 Peptide Pur...
-
Melanotan II (MT-II) CAS 121062-08-6 Peptide Pu...
-
Cetrorelix Acetate CAS 130143-01-0 GnRH Antagon...
-
Octreotide Acetate CAS 83150-76-9 Peptide Purit...


